原文标题:《脂质体递送系统:设计优化和当前应用(Liposomal Delivery Systems: Design Optimization and Current Applications)》(有删节)

作者简介
Amr Selim Ahmed Ali Abu Lila
于2005年在埃及扎加齐格大学药学院获得药剂学硕士学位,并于2010 年获得日本德岛大学药学研究生院药物生命科学博士学位。2011年至2013年,于日本德岛大学药学研究生院任日本学术振兴学会外籍特别研究员博士后。现任埃及扎加齐格大学药学院副教授。已发表超25篇同?评审科学期刊论文、4 篇评论文章和5个书籍章节。目前的研究重点是使用脂质体药物递送系统将选择性靶向化疗药物或基因导入肿瘤组织的抗癌疗法。
关键词:给药系统;基因治疗; 免疫疗法;脂质体;聚乙二醇化;治疗诊断学
Key words:drug delivery system; gene therapy; immunotherapy; liposome; PEGylation; theranostics.
摘要:脂质体,是一种封闭的磷脂双层囊泡系统,作为一种具有巨大潜?的药物载体,在过去的30年?受到了广泛的关注。
Abstract: Theliposome, a closed phospholipid bilayered vesicular system, has received considerable attention as a pharmaceutical carrier of great potential over the past 30 years.
脂质体封装亲水性和疏水性药物的能?,以及其生物相容性和生物降解性,使脂质体成为药物递送领域的热门载体。
The ability of liposomes to encapsulate both hydrophilic and hydrophobic drugs, coupled with their biocompatibility and biodegradability, make liposomes attractive vehicles in the field of drug delivery.
此外,远程载药、触发释放脂质体、配体靶向脂质体、药物组合脂质体等重大技术进步,使得脂质体作为抗癌、生物活性分子、诊断、治疗剂等的递送载体在多种领域得到广泛应用。
In addition, great technical advances such as remote drugloading, triggered release liposomes, ligand-targeted liposomes, liposomes containing combinations of drugs, and so on, have led to the widespread use of liposomes in diverse areas as delivery vehicles for anti-cancer, bio-active molecules, diagnostics, and therapeutic agents.
在这篇综述中,我们总结了脂质体系统的设计优化以及脂质体作为有效递送系统的宝贵应用。
In this review, we summarize design optimization of liposomal systems and invaluable applications of liposomes as effective delivery systems.
1. 简介
自Alec Bangham于1964年首次发现磷脂可以在水性系统中形成封闭的双层结构以来, 脂质体已成为医学领域最有前途的药物靶向工具之一。
Since the pioneering observation of Alec Bangham in 1964 that phospholipids in aqueous systems can form closed bilayered structures, liposomes have emerged as one of the most promising tools for drug targeting in medical fields.
脂质体的固有优势,如生物相容性、低毒性、高负载能?和可控的释放动?学, 激发了它们作为药物载体的有效使用,并成就了脂质体制剂用于治疗真菌感染的首个上市批准:Ambisome?,一种封装抗真菌剂两性霉素B的传统脂质体。
The inherent advantages of liposomes, such as biocompatibility, low toxicity, high loading capacity, and controllable release kinetics, have inspired their efficient use as drug carriers and triggered the first approval of a liposomal formulation for the treatment of fungal infections: Ambisome?, a conventional liposomes encapsulating the antifungal agent, amphotericin B.
然而,尽管取得了这一成功,这种传统脂质体的体内命运的效果却令人沮丧。传统脂质体倾向于彼此融合和/或聚集,这会导致脂质体的有效负载随着时间推移不完全释放。
However, despite this success, the in vivo fate of such conventional liposomes was dismal. Conventional liposomes tend to fuse and/or aggregate with each other resulting in immature release of liposomal payload over time.
此外,传统脂质体通过被单核吞噬细胞系统 (MPS)的细胞摄取,会经历快速全身清除。
In addition, conventional liposomes underwent rapid systemic clearance via their uptake by the cells of mononuclear phagocyte system(MPS).
为了克服这些限制,常用的表面改性策略是在传统脂质体的表面修饰具有惰性、生物相容性的亲水性聚合物, 例如聚乙二醇(PEG)。
To overcome these limitations, surface-modification strategies were adopted by coating the surface of conventional liposomes with inert, biocompatible hydrophilic polymers, such as polyethylene glycol (PEG).
这种亲水性聚合物通过在脂质体表面形成保护层,为脂质体表面赋予了空间稳定性,并减缓了调?素对脂质体的识别和脂质体的后续清除。
Such hydrophilic polymer confers steric stabilization to the liposomal surface via formation of a protective layer over the liposome surface and slows down liposome recognition by opsonins, and therefore, subsequent clearance of liposomes.
因此,隐形技术、聚乙二醇化,已促成了多种临床批准的脂质体制剂的开发,例如用于癌症治疗的Doxil?、 Caelyx?和Myocet?。
As a result, stealth technology, PEGylation, has led to the development of several clinically approved liposomal formulations such as Doxil, Caelyx, and Myocet? used for the treatment of cancers.

然而,尽管聚乙二醇化脂质体的药代动力学得到了改善,但其所欠缺的靶向选择性,大大限制了聚乙二醇化脂质体在许多临床环境中的治疗潜力。因此,多种工程策略已被应用于改善脂质体的体内性能。
Nonetheless, despite the improved pharmacokinetics of PEGylated liposomes, the lack of target selectivity substantially restricted the therapeutic potential of PEGylated liposomes in many clinical settings. Accordingly, several engineering strategies have been applied to improve the in vivo performance of liposomes.
这些策略包括附着定点表面配体,如抗体(免疫脂质体)、正电荷(阳离子脂质体)或肽(肽靶向脂质体);或利用靶组织内的固有生理条件,如温度升高或pH改变(图1),以产生刺激响应脂质体,如热敏脂质体和pH敏感脂质体。
These strategies include either the attachment of site-directed surface ligands, such as antibodies (immunoliposomes),positive charge (cationic liposomes), or a peptide (peptide-targeted liposomes), or exploiting the inherent physiological conditions in the target tissue, such as elevated temperature or alteration in pH (Fig. 1) so as to produce stimuli-responsive liposomes such as thermo-sensitive liposomes and pH-sensitive liposomes.
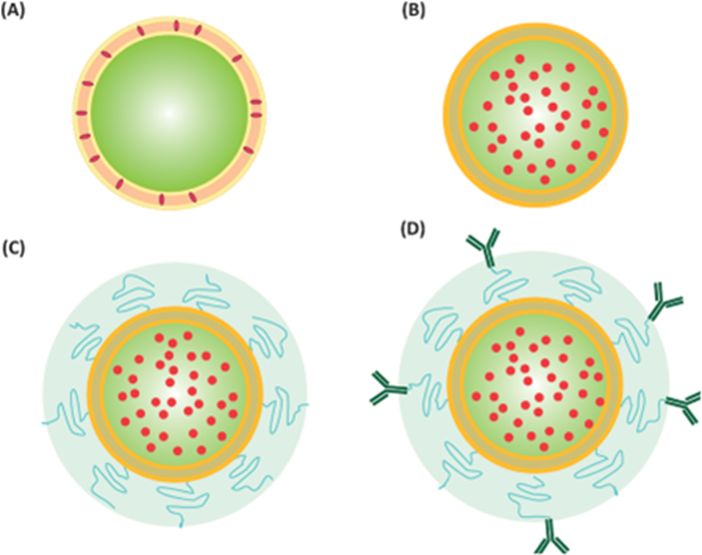
图 1. 不同脂质体制剂的结构
(A) 包埋脂溶性药物的传统脂质体;(B) 包埋水溶性药物的传统脂质体;(C) 空间稳定脂质体;(D) 含有水溶性药物的配体靶向脂质体。图片修改自:Abu Lila A, IshidaT, AllenT. 《脂质体纳米药物,纳米生物医学研究手册》(Torchilin V 编辑)。世界科学出版社,2014 年。
Fig. 1. Structures of Different Liposomal Preparations
(A) Classical liposome encapsulating lipid soluble drugs; (B) classical liposome encapsulating aqueous soluble drugs; (C) sterically stabilized liposomes; (D) ligand-targeted liposome containing an aqueous soluble drug. Figure modified from: Abu Lila A, IshidaT, AllenT. “Liposomal Nanomedicine, Handbook of Nano biomedical Research (Torchilin V, ed.). World Scientific Publishing, 2014.
最近的几份报告探讨了脂质体在新型结构配方或当前临床进展的具体方面。在本综述中,我们强调了脂质体优化设计面临的一些固有问题。我们还特别关注脂质体作为高效输送系统的最新应用。
Several recent reports have discussed specific aspects of liposomes regarding novel structural formulations or current clinical progress. In this review, we highlight some inherent liposomal problems facing their optimized design. We also place special focus on recent applications of liposomes as efficient delivery systems.
2. 脂质体递送系统的设计优化
2.1. 提高包封效率
众所周知,将药物装载到纳米颗粒(包括脂质体)中,通过允许将足够浓度的被包裹药物选择性地递送到作用部位,同时抑制其递送到非靶(正常)组织,可以增加被包裹药物的治疗比率[一般称治疗指数(therapeutic index),指半数致死量(LD50)与半数有效量(ED50)的比值,可在一定程度上反映药物的安全性——译者按]。
Drug loading into nanoparticles, including liposomes, is known to increase the therapeutic ratio of the entrapped drug by permitting selective delivery of adequate concentrations of the entrapped drug to the site of action while restraining its delivery to non-target (normal) tissues.
然而,许多脂质体制剂的治疗效果在各种实验/临床环境中差强人意,(至少有一部分)原因是由于药物包封效率低。
However, the therapeutic impact of many liposomal formulations was compromised in various experimental/clinical settings by, at least in part, low drug entrapment efficiency.
脂质体的商业价值,被主动“远程”药物载药方法的发明所加强,该方法允许以很高的药物/脂质比(包封效率高达90%),包封几种弱碱性或弱酸性药物。
The commercial impact of liposomes is strengthened by the invention of active “remote” drug loading methods, allowing the encapsulation of several weak base or weak acid drugs with very high drug-to-lipid ratios (encapsulation efficiency up to 90%).
在主动负载法中,通过操纵脂质体内部水相室的pH值或化学成分,可以使药物在脂质体中高效保留。该方法被广泛应用于多柔比星、柔红霉素和长春新碱等药物的高效包封。
In the active loading method, the pH or chemical composition of the internal aqueous compartment of the liposomes is manipulated to allow efficient retention of drugs within the liposomes. This method was widely applied for the efficient entrapment of drugs such as doxorubicin, daunorubicin, and vincristine.
尽管上述策略在某些情况下已被证明是有利的,但对于许多高度疏水性或缺乏电离基的药物(例如紫杉醇或环丙沙星),主动负载并不被认为是一种通用方法,它们无法被远程装载到脂质体中。
Although the above strategy has proven advantageous in certain circumstances, active loading is not considered a universal method with many drugs that are highly hydrophobic or lack an ionizable group, such as paclitaxel or cipro oxacin, which cannot be remotely loaded into liposomes.
尽管如此,Sur等人最近汇报了一种创新方法,可以主动负载?含可电离基团的疏水性化疗药物。
Nonetheless, Sur et al. have recently reported an innovative method to allow the active loading of hydrophobic chemotherapeutics devoid of ionizable groups.
他们采用表面带有可电离基团的改性环糊精。这些环糊精的“口袋”可以封装疏水性药物,并使用简单的pH梯度将它们运送到传统脂质体的双层膜中。
They employed modified cyclodextrins with ionizable groups on their surfaces. The “pockets” of these cyclodextrins can encapsulate hydrophobic drugs and ferry them across the bilayer membrane of conventional liposomes using simple pH gradients.
他们强调,通过这种独特方法达到的药脂比(在脂质体中的药物分子与脂质分子的摩尔比或重量比,用于衡量脂质体药物载药效果的指标——译者按),比通常通过被动装载制成的脂质体高 1000 倍以上。
They emphasized that the drug: lipid ratios achieved through this unique approach was >1000-fold higher than those commonly achieved through passive loading.
2.2. 药物释放速率控制
众所周知,脂质体中包埋的药物,只有在释放后才具有生物利用度。因此,充分优化脂质体有效载荷的释放速率,被认为是决定脂质体系统整体治疗效果的关键性因素。
It is well recognized that the entrapped drug within liposomes is not bioavailable until it is released. Therefore optimizing the release rate of liposomal payload is considered a crucial determinant for the overall therapeutic efficacy of liposomal systems.
一般来说,脂质体应在足够长的时间内,以适当的速率,在靶组织内递送足够浓度的、具有生物活性的药物,同时在转运到靶部位的过程中保留药物(即,?会过早地释放药物释放)。
As a general rule, liposomes should grant the delivery of adequate concentration of bioavailable drug within the target tissue, at an appropriate rate, for a sufficient period of time, while retaining the drug during transit to the target site (i.e., no premature release).
Kim等人报道,尽管顺铂隐形脂质体制剂在小鼠肿瘤模型中具有有效的瘤内递送,但其并未发挥任何治疗潜力。
Kim et al. have reported that despite the efficient intra tumor delivery of stealth liposomal formulation of cisplatin in mouse tumor model, the liposomes failed to exert any therapeutic potential.
他们将这种矛盾的结果,归因于脂质体未能在肿瘤组织中释放最低细胞毒性浓度的顺铂。
They attributed such con?icting results to the failure of liposomes to release a minimum cytotoxic concentration of cisplatin at the tumor tissue.
在原位恶性间皮瘤肿瘤模型中,我们在胸膜内给药载有培美曲塞的含胆固醇脂质体后,观察到了类似的结果。
We observed similar results after intrapleural administration of cholesterol containing liposomes loaded with pemetrexed in orthotopic malignant mesotheliomal tumor model.
脂质体的药物释放受脂质体膜的组成和包裹药物的物理化学性质的影响。
Drug release from liposomes was demonstrated in?uenced by liposomal membrane composition and the physicochemical properties of the encapsulated drug.
向脂质体膜中加入胆固醇,可以使膜结构具有刚性,并从液相磷脂双分子层转换为固相双分子层,似乎会导致药物从脂质体中的释放减少。
Inclusion of cholesterol into the liposomal membrane, which has a membrane rigidizing effect,and switching from a fluid phase phospholipid bilayer to a solid phase bilayer were shown to reduce the release of drugs from liposomes.
辛醇/缓冲液分配系数极低的药物在脂质体中停留时间较长,而分配系数在-0.3~1.7之间的药物释放较快。有趣的是,药脂比对药物释放的影响,也取决于包封特定药物。
Drugs with extremely low octanol/buffer partition coefficients exhibited prolonged liposomal retention whereas molecules with partition coefficients ranging from -0.3 to 1.7 were, in contrast, rapidly released.Interestingly, the effect of drug-to-lipid ratio on drug release is also dependent on the specific drug entrapped.
就多柔比星而言,药脂比越高,药物保留半衰期越短。相反,长春新碱和伊立替康在药脂比更高的脂质体中保留时间更长;当药脂比从 0.05 增加到 0.6(重量比)时,释放半衰期延长了 10 倍。
In the case of doxorubicin, a higher drug-to-lipid ratio resulted in decreased drug retention half-life. In contrast, vincristine and irinotecan were retained longer in liposomes with higher drug-to-lipid ratios; a 10-fold increase in release half-life was observed as drug-to-lipid ratio was increased from 0.05 to 0.6 (w/w).
2.3. 操纵脂质体的体内命运
除了对MPS(巨噬细胞)参与的疾病(如利什曼病)的疗法以外,巨噬细胞快速摄取脂质体,并随之将其从循环中清除的现象,是“传统”脂质体在药物递送系统领域潜在应用的主要挑战。
Except for the treatment of diseases where there was an MPS involvement, e.g., leishmaniasis, the rapid liposomal uptake by the macrophages and their consequent removal from circulation represented a major challenge against the potential use of “classical” liposomes as drug delivery systems.
因此,人们一直在努力提高脂质体在全身给药后的药代动力学特性。
Accordingly, many attempts have been devoted to enhance their pharmacokinetic characteristics following systemic administration.
最初,人们发现通过操纵脂质体的理化性质,如大小、流动性、净表面电荷和脂质双层膜的填充,不仅能影响脂质体的物理稳定性,还会影响 MPS 细胞对脂质体的摄取。
Initially, manipulation of the physicochemical properties of liposomes, such as size, ?uidity, net surface charge, and packing of the lipid bilayers, has been found to affect not only liposomal physical stability but also their uptake by cells of the MPS.
与中性和/或小尺寸脂质体相比,带电脂质体和/或大尺寸脂质体从全身循环中清除的速度更快。
Charged liposomes and/or large-size liposomes are cleared from the systemic circulation more rapidly than neutral and/or small-size liposomes.
此外,使用饱和磷脂或加入胆固醇,可增加脂质双层中磷脂的填充,进而减少MPS细胞对脂质体的摄取。
In addition,the use of saturated phospholipids or the incorporation of cholesterol, which increases the packing of phospholipids in the lipid bilayer, reduces liposomal uptake by cells of the MPS.
然而,这种方法无法完全克服传统脂质体与血清成分的结合,只能略微降低 MPS 对脂质体的吸收。
However, this approach cannot fully overcome the binding of classical liposomes with serum components and only slightly decreased MPS uptake of liposomes.
一种早期的替代方法,是预先注射大剂量的“空的”传统脂质体,以饱和MPS细胞的吞噬摄取能力。
An early alternative approach was to administer large pre-doses of “empty” classical liposomes to saturate the phagocytic uptake capacity of cells of the MPS.
此后出现了一种更吸引人的提高脂质体药代动力学的方法,即在脂质体表面修饰惰性的、生物相容性的亲水聚合物,如神经节苷脂 GM1、磷脂酰肌醇或脂质共轭 PEG。
Thereafter, a more fascinating approach to enhance the pharmacokinetics of liposome was via liposomal surface decoration with inert, biocompatible hydrophilic polymers, such as ganglioside GM1, phosphatidylinositol, or lipid- conjugated PEG.
这些聚合物通过在脂质体表面形成亲水性保护层发挥作用,在空间上阻碍血清调理素与脂质体表面的结合,从而减缓脂质体的全身清除。
These polymers act by forming a hydrophilic protective layer over the liposome surface that sterically hinders the attachment of serum opsonins to the liposomal surface and accordingly slows down the systemicclearance of liposomes.
通过减少MPS摄取,长循环脂质体通过被动靶向效应增加了在靶组织内积累的机会。
By reducing MPS uptake, longer-circulating liposomes have enhanced opportunity to accumulate inside the target tissue via what is called passive targeting.
肿瘤血管的特殊透过性,使得脂质体可以通过高通透性和滞留(EPR)效应,优先积聚到肿瘤组织中,这种现象在实体瘤中表现得非常明显。
This phenomenon is highly manifested in solid tumors where the leaky nature of tumor vasculature triggers the preferential accumulation of liposomes into the tumor tissue via a process known as enhanced permeation and retention (EPR) effect.
由于这一成功,长循环脂质体目前已被应用于临床实践。
As a result of this success, long-circulating liposomes are currently adopted in clinical practice.
2.4. 加强脂质体有效载荷的细胞内输送
尽管脂质体作为药物和/或生物大分子的有效载体被广泛应用,但其临床效果却因为封装的有效载荷在胞内传递效率的低下而严重受损。
Despite the widespread use of liposomes as attractive delivery vehicles for drugs and/or biomolecules, their clinical efficiency was severely compromised by the inefficient intracellular delivery of encapsulated payload.
生物膜具有亲脂性,这限制了脂质体直接进入靶细胞,使其只能通过吞噬作用和/或内吞作用被细胞吞噬。
The lipophilic nature of the biological membranes restricts direct entrance of liposomes into target cells. Instead, liposomes are engulfed into cells via phagocytosis and/or endocytosis.
然而,受体介导的内吞作用导致脂质体进入溶酶体,在溶酶体和内体的酸性和富酶环境中,脂质体很容易被降解。
Nonetheless, receptor-mediated endocytosis of liposomes results in their lysosomal delivery, where they become vulnerable to degradation by the acidic and enzyme-rich environment of the endosomes and lysosomes.
最近,人们非常关注于验证不同的策略来绕过内吞途径(内吞逃逸),从而增强包裹药物的治疗效果。
Recently, much attention has been devoted to verifying different strategies to bypass the endocytic pathway (endosomal escape) and, thereby, enhancing the therapeutic efficacyof encapsulated drug.
pH敏感肽或阳离子脂质的应用,被认为是一种有效的策略,可在脂质体通过吞噬和/或内吞作用被摄取后,扰乱内体/溶酶体膜,以增强脂质体包载物的胞质传递。
The use of pH-sensitive peptides or cationic lipids is considered an efficient strategy to enhance the cytoplasmic delivery of liposomal cargo by disrupting the endosome/lysosome membrane following liposomal uptake by phagocytosis and/or endocytosis.
另外,据报道,将膜融合性脂质加入脂质体和/或使用穿膜蛋白或多肽(CPPs),可以增强脂质体包载物以非内吞作用方式进行胞质传递。
Alternatively, incorporation of fusogenic lipids into liposomes and/or the use of cell-penetrating proteins or peptides(CPPs), proteins and peptides that can translocate through the cellular membranes, reportedly may enhance cytoplasmic delivery of liposomal cargo via an endocytosis-independent manner.
不过,这些非内吞途径不如利用内吞的途径那么热门(图 2)。
However, these non-endocytotic pathways are less prominent than those using endocytosis (Fig. 2).
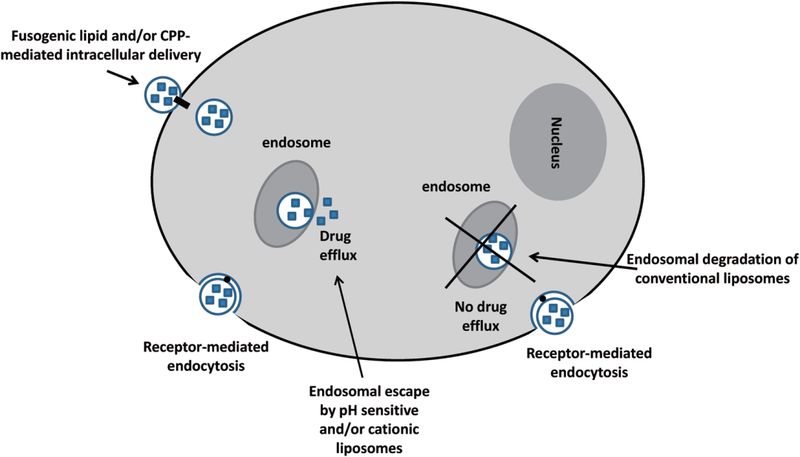
图 2. 脂质体包载物细胞内输送示意图
Fig. 2. Schematic Representation of Intracellular Delivery of Liposomal Cargo
3. 脂质体的抗肿瘤应用
在临床上,脂质体已经展示了广泛的应用。
Liposomes have demonstrated a wide range of applications in clinical settings.
越来越多的文献已经论证了将多种药物配制成脂质体的可行性,与游离药物相比,脂质体可提高药物疗效和/或降低封装药物的全身毒性。
A mounting body of literature has defined the viability of formulating a wide range of drugs in liposomes taking advantage of improved therapeutic efficacy and/or reduced systemic toxicity of the encapsulated drug compared with the free counterpart.
一般来说,通过封装改变脂质体药物的药代动力学,可达到药物血液循环延长、生物利用度提高和/或在疾病部位优先蓄积的效果。
Generally, alteration of the pharmacokinetics of liposomal drugs, via encapsulation,can lead to prolonged blood circulation characteristics, enhanced drug bioavailability, and/or preferential accumulation in disease sites.
小分子药物,尤其是化疗药物的递送,是脂质体递送系统的最早的临床应用之一。
Delivery of small molecule therapeutics, particularly chemotherapeutic agents, was one of the first clinical applications of liposomal delivery systems.
传统化疗药物不仅会杀死病变细胞,也会滥杀快速生长的健康细胞,从而对血细胞、毛囊等组织以及肠黏膜细胞产生严重的副作用,并对临床药物剂量和给药频率造成限制。
Conventional chemotherapeutic agents may indiscriminately kill not only diseased cells but also rapidly growing healthy cells, leading to severe side effects on tissues such as blood cells and hair follicles and on cells lining the intestinal mucosa, and in?icts practical limits on the drug dose and dosing frequency.
利用肿瘤组织的病理生理条件(如肿瘤血管渗漏),将药物封装在纳米载体中,是一种非常有价值的抗癌策略。
Exploiting the patho-physiological conditions of tumor tissue, such as leakytumor vasculature, along with drug encapsulation within a nanocarrier represented an invaluable strategyfor combating cancer.
传统脂质体或 PEG 化脂质体,利用 EPR 效应介导的被动靶向策略,可将包裹的药物优先积聚到肿瘤组织中,从而在提高疗效的同时将副作用降至最低。
Classical liposomes or PEGylated liposomes use a passive targeting strategy, mediated by the EPR effect, preferentially to accumulate the encapsulated drug into the tumor tissue and, thereby, enhance its therapeutic efficacy while minimizing its side effects.
多柔比星(DXR)的 PEG 化脂质体制剂 Doxil 的临床应用,就是一个出色的例子,多柔比星是一种蒽环类抗癌药,对多种实体瘤有很强的疗效。
A brilliant example is the clinical use of Doxil, a PEGylated liposomal formulation of doxorubicin (DXR), an anthracycline anticancer agent active against a wide variety of solid tumors.
据报道,多柔比星的脂质体封装可提高封装药物的治疗效果,同时降低蒽环类药物诱发心脏毒性的风险,而这种风险可能会限制游离药物的临床应用。
Liposomal encapsulation of doxorubicin was reported to enhance the therapeutic efficacy of the encapsulated drug, in tandem with reducing the risk of anthracycline induced cardiotoxicity, which potentially limits the clinical use of the free drug.
1995 年,Doxil? 获得批准后,一些细胞毒性药物的脂质体制剂开始被应用于临床,而更多的抗癌药物脂质体制剂目前正处于不同的临床试验阶段。
After the approval of Doxil in 1995, a number of cytotoxic agent-containing liposomes have emerged for clinical use whereas more liposomal formulations of anti-cancer agents are currently in various stages of clinical trials.
尽管脂质体抗癌药物的被动靶向递送取得了上述进展,但许多报告强调,这种靶向策略无法确保在肿瘤组织内充分递送最低治疗浓度的封装药物,从而导致治疗失败。
Despite the aforementioned advances achieved by passive targeting-mediated delivery of liposomal anticancer drugs, many reports have emphasized the failure of such targeting strategy in ensuring the adequate delivery of a minimum therapeutic concentration of encapsulated drug within tumor tissue, resulting in treatment failure.
另外,人们还广泛研究了一种主动的 "配体介导 "靶向方法,这种方法能够改善封装药物在肿瘤组织内的细胞内输送。
Alternatively, an active “ligand-mediated” targeting approach has been extensively investigated for its ability to improve intracellular delivery of encapsulated drug within tumor tissue.
人们研究了多种分子,包括肽、抗体、蛋白质、带电分子和一些低分子量配体(如叶酸),以及基于核酸的适配体,它们与脂质体结合使用,可提高抗癌治疗效果。
A variety of molecules, including peptides, antibodies, proteins, charged molecules, and some low-molecularweight ligands, such as folate, and nucleic acid-based aptamers, have been studied in conjunction with liposomes for enhanced anticancer treatment.
利用肿瘤微环境的特定特征(如细胞外 pH 值降低和细胞外蛋白质模式改变)也能增强肿瘤靶向性。
Enhanced tumor targeting can also be attainedvia exploiting specific features in the tumor microenvironment such as lowered extracellular pH and an alteredpattern of extracellular proteins.
例如,Zhu 等利用肿瘤微环境中基质金属蛋白酶 2 水平的升高,通过将抗核糖体单克隆抗体(mAb 2C5)与 PEG 化脂质体共轭,改善了负载药物的癌细胞特异性递送。
For example, Zhu et al. exploited elevated levels of matrix metalloprotease 2 in the tumor microenvironment to improve cancer cell-specific delivery of loaded drugs via conjugating the anti-nucleosome monoclonal antibody (mAb 2C5) to PEGylatedliposomes.
另一种策略是利用外部刺激(如磁场、改变温度的超声波和光)来改善包封抗癌药物向肿瘤的输送。
Another strategyis to use external stimuli, such as a magnetic field, altered temperature ultrasound and light to improve delivery of encapsulated anticancer agents to tumors.
4. 结论
开发脂质体作为治疗分子的载体是一个不断发展的研究领域。
The development of liposomes as carriers for therapeutic molecules is an ever-growing research area.
操纵这些纳米载体的固有特性的可行性,使它们能够成为多种材料(药物、蛋白质、肽、核酸等)的多功能载体,并拓宽了它们在许多临床环境中的潜在用途。
The possibility of manipulating the inherent characteristics of these nanocarriers makes them versatile carriers for a wide range of materials (drugs, proteins, peptides, nucleic acids, and so on) and widens their potential use in many clinical settings.
此外,脂质体还具有共封装治疗剂和诊断剂的能力,这为脂质体递送系统作为治疗平台的新型应用铺平了道路。
Furthermore, the ability of liposomes to co-encapsulate both therapeutic and diagnostic agents pave the way for a novel application of liposomal delivery systems as theranostic platforms.
然而,合理地设计实现治疗目标的方法,可能是未来开发更复杂脂质疗法的决定性步骤。
However, a rational design approach to achieve therapeutic objectives might represent the rate-determining step in the development of more sophisticated lipid-based therapeutics in the future.








